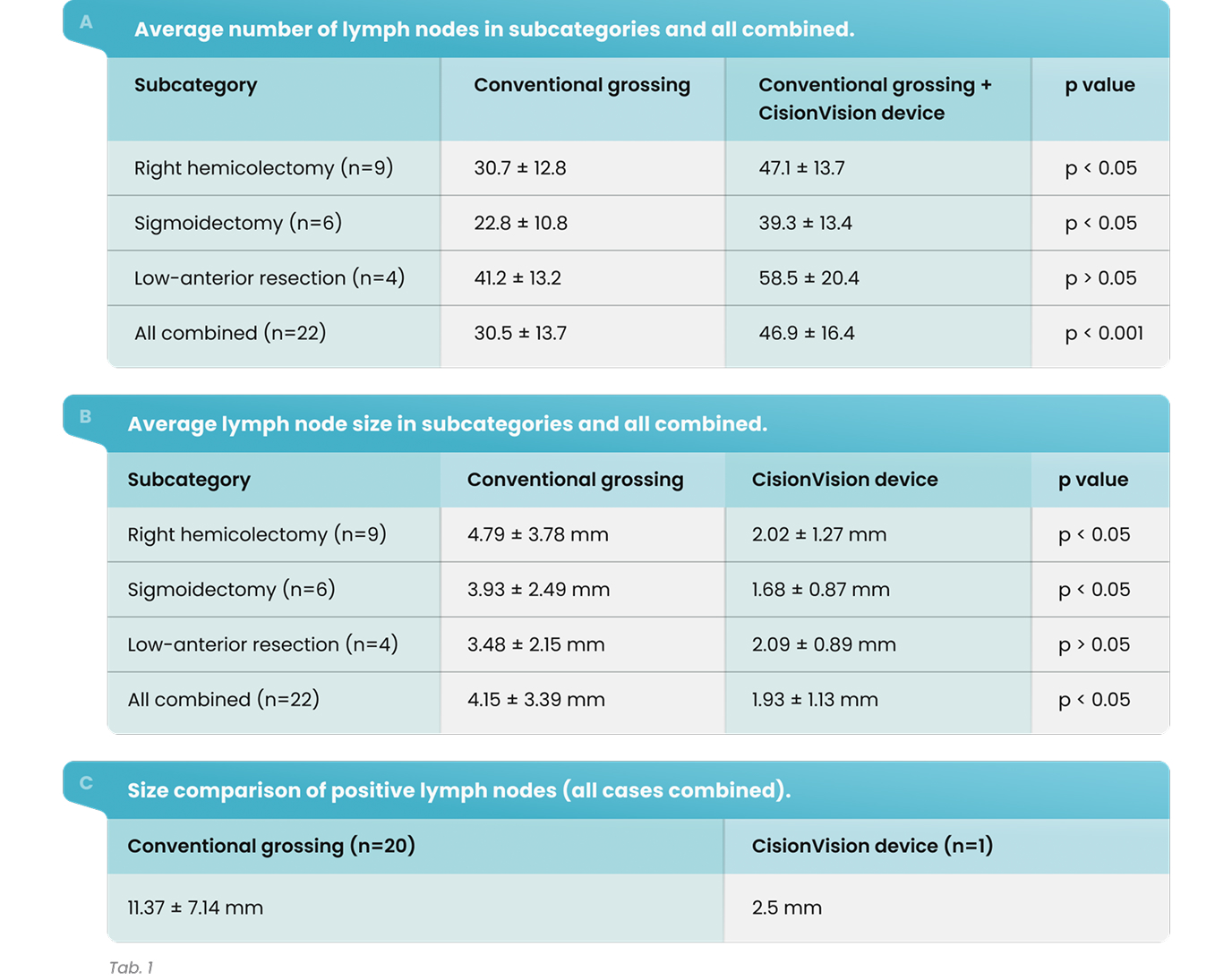
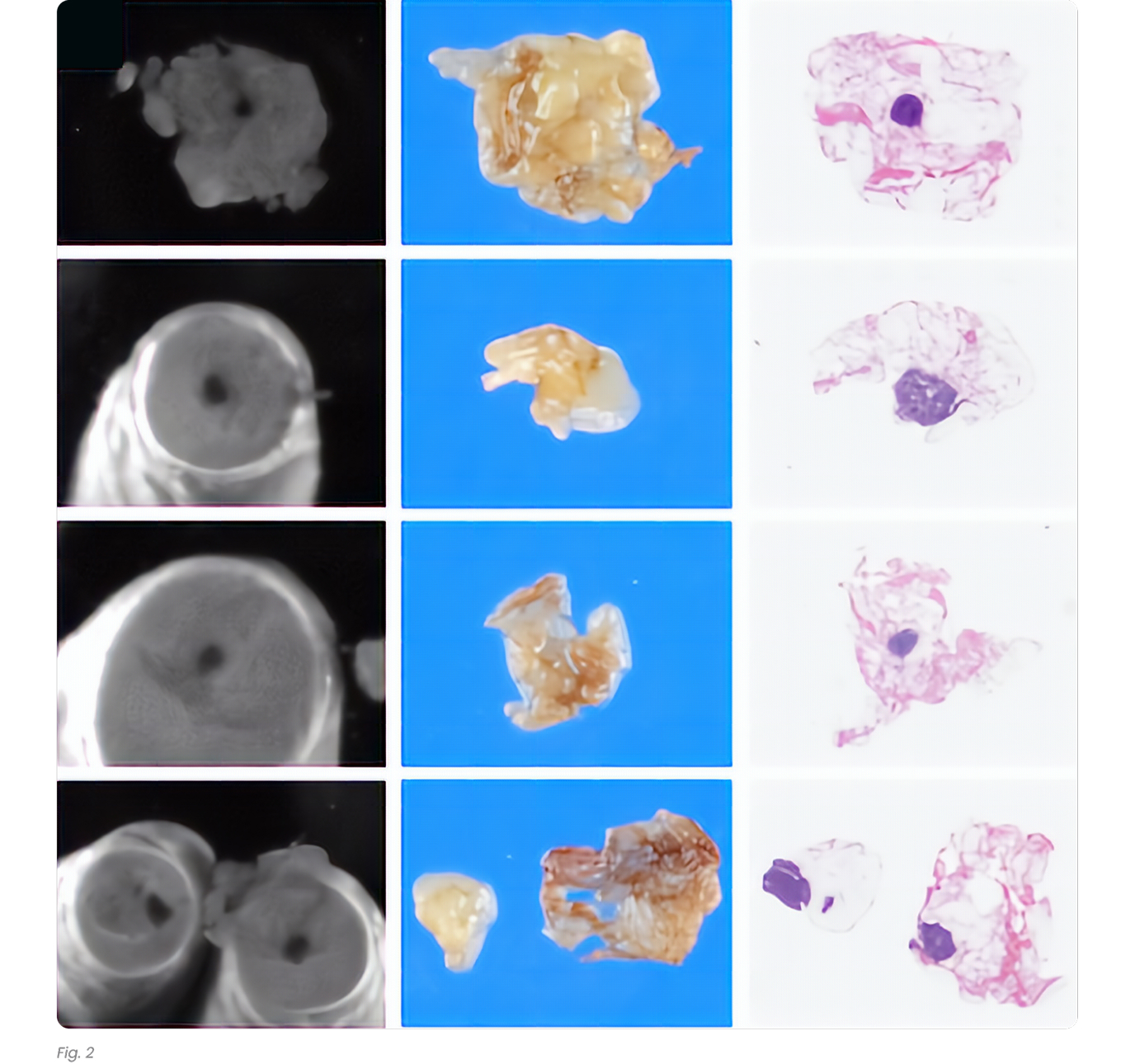
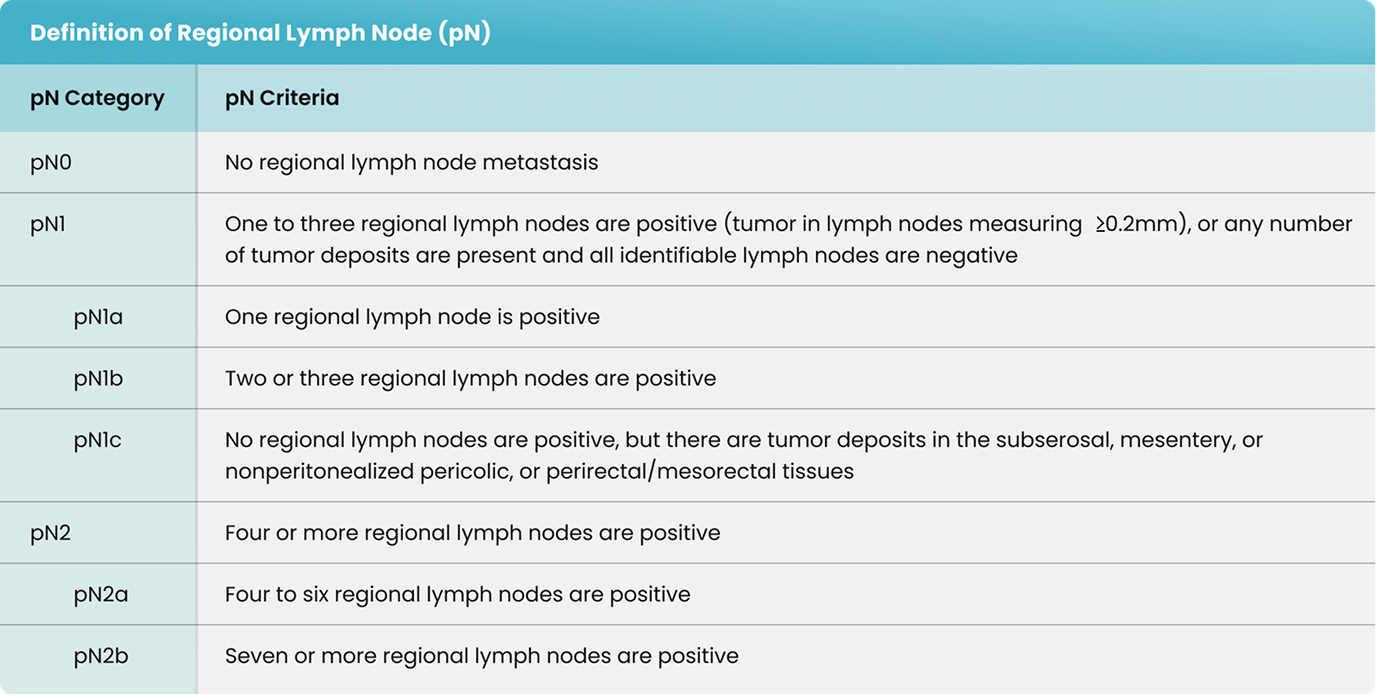
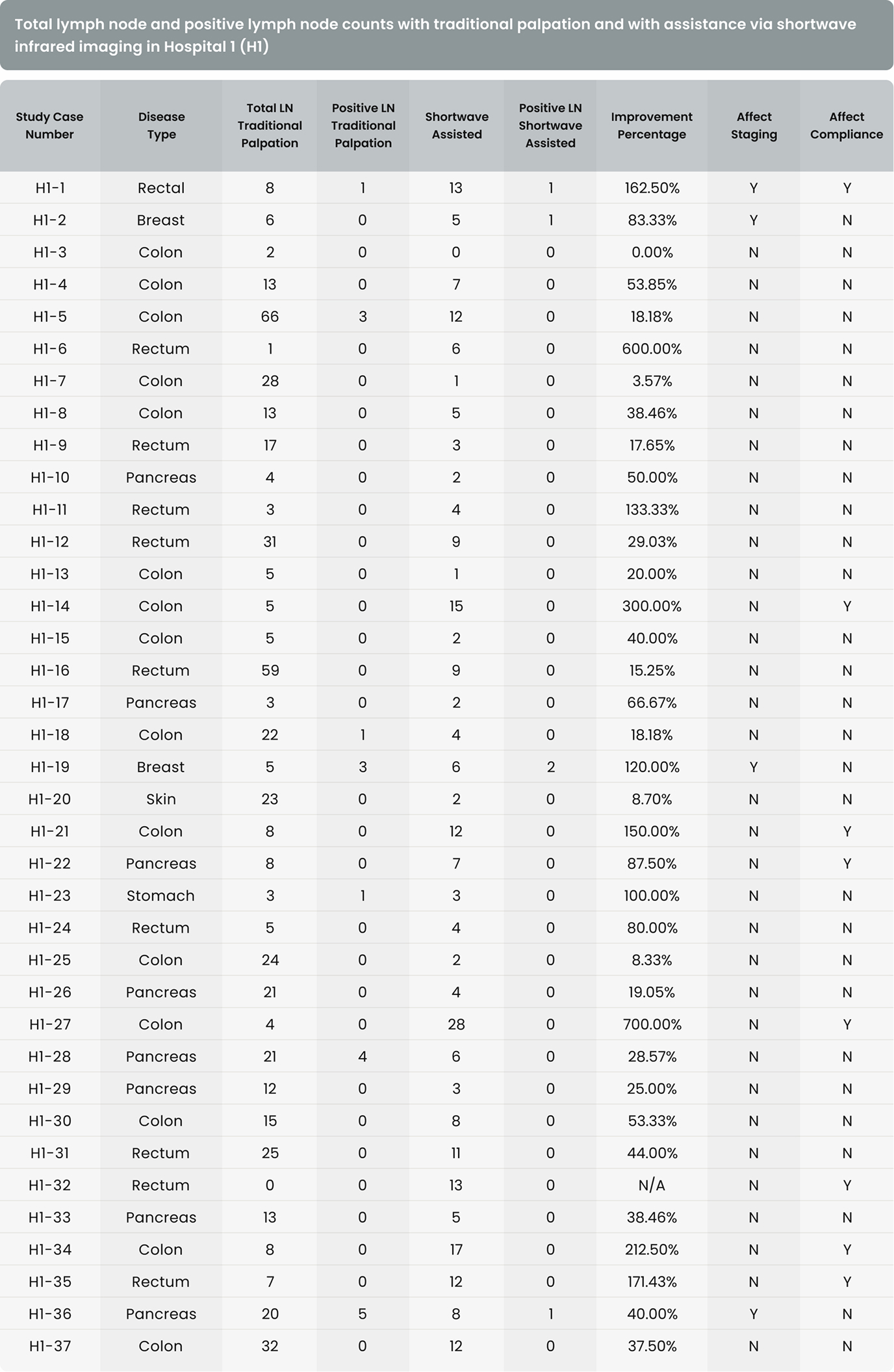
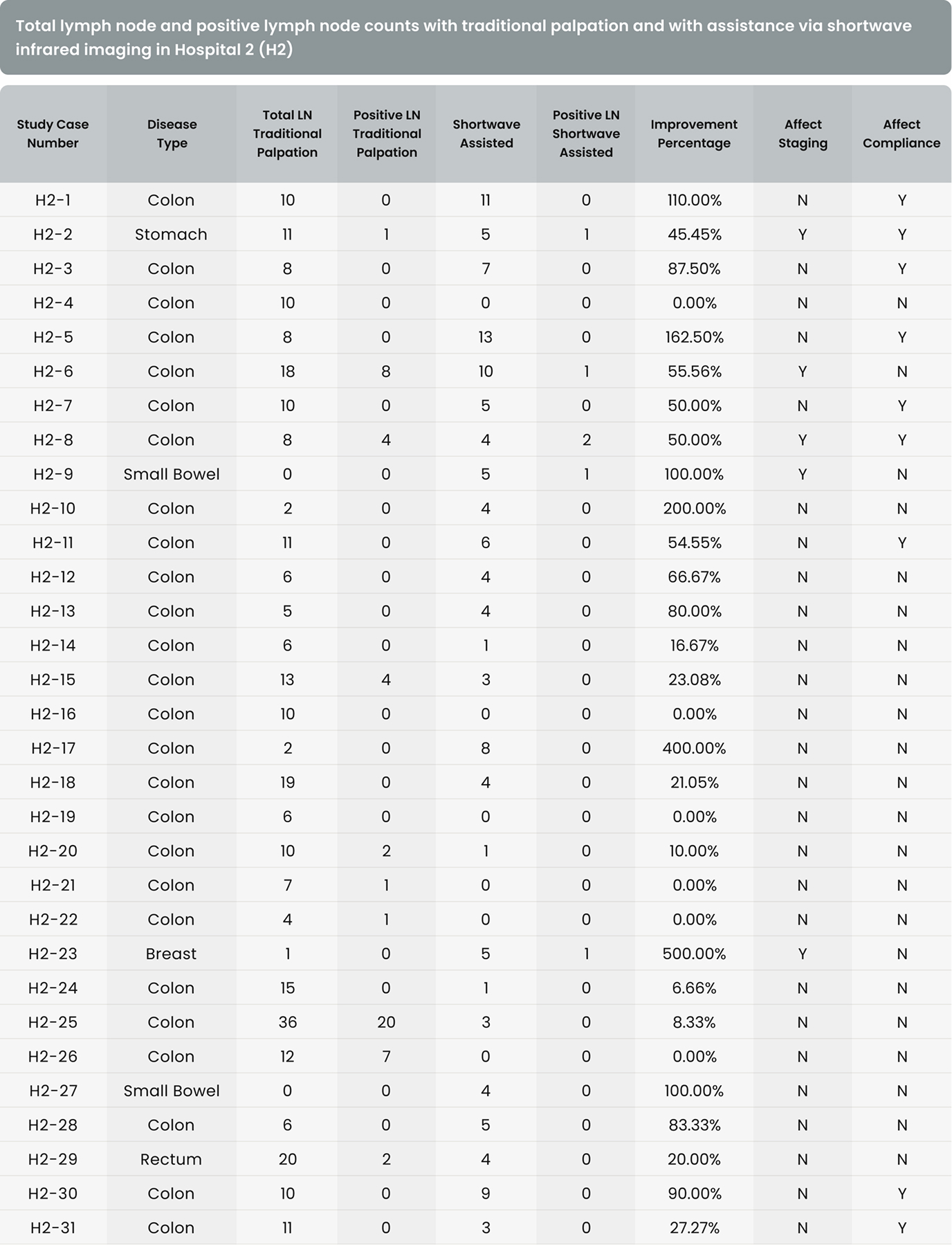
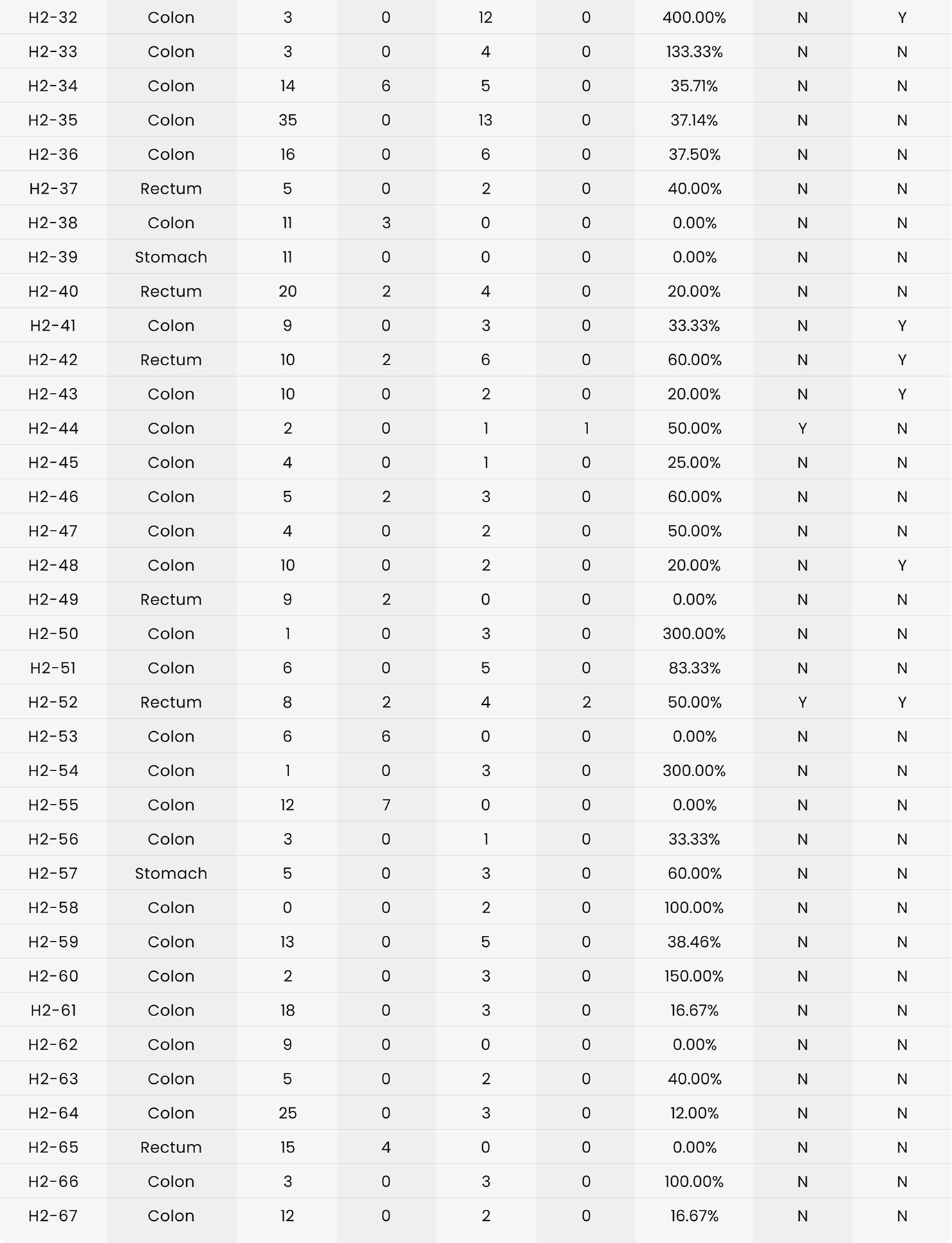
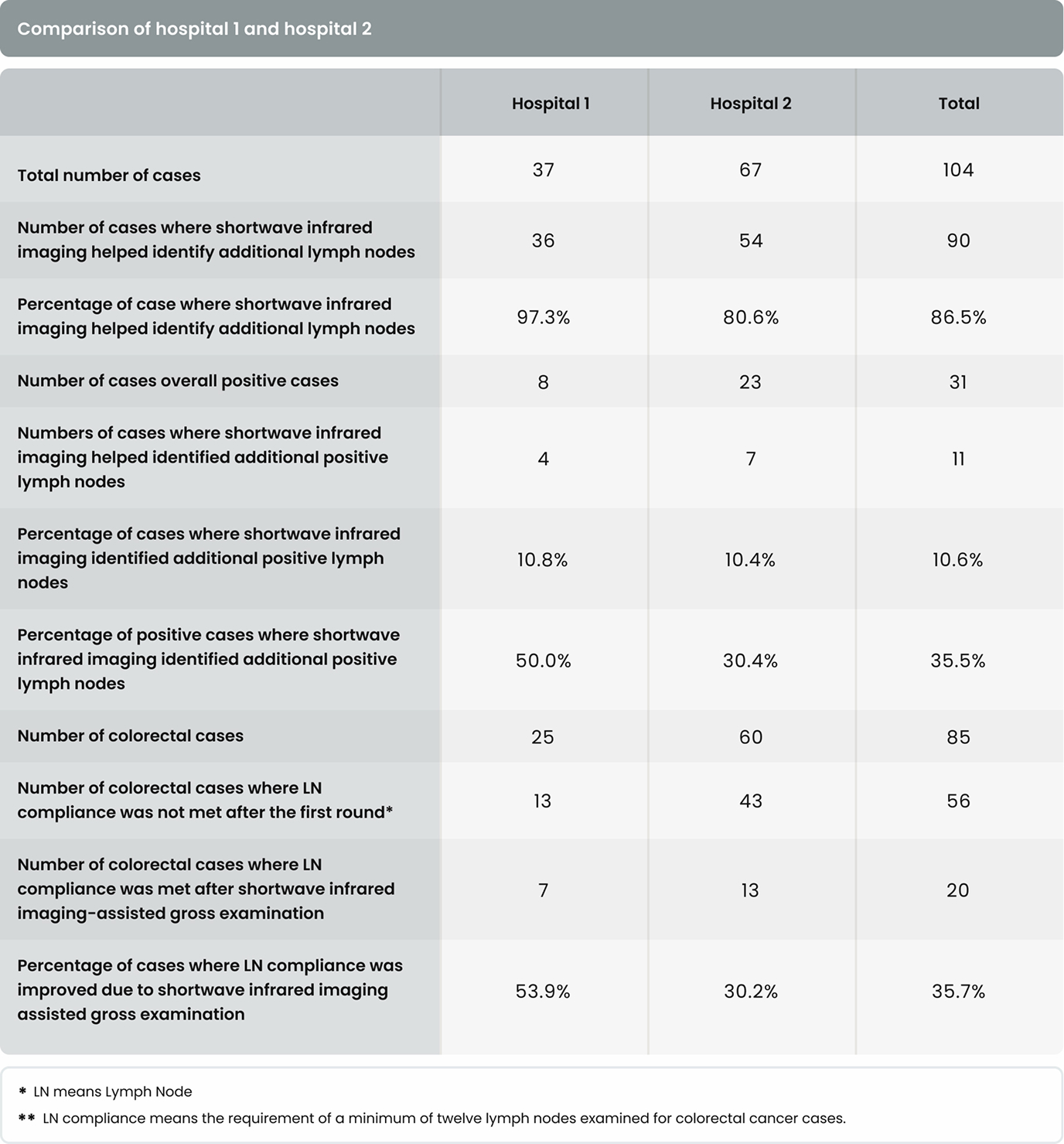
As shown in Table 4, in 36 of the 37 cases (97.3%) in Hospital 1 and 54 of the 67 cases (80.6%) in Hospital 2, additional lymph nodes were identified with the assistance of shortwave infrared imaging. Overall, in 90 of the 104 cases (86.5%), additional lymph nodes were identified with the assistance of shortwave infrared imaging.
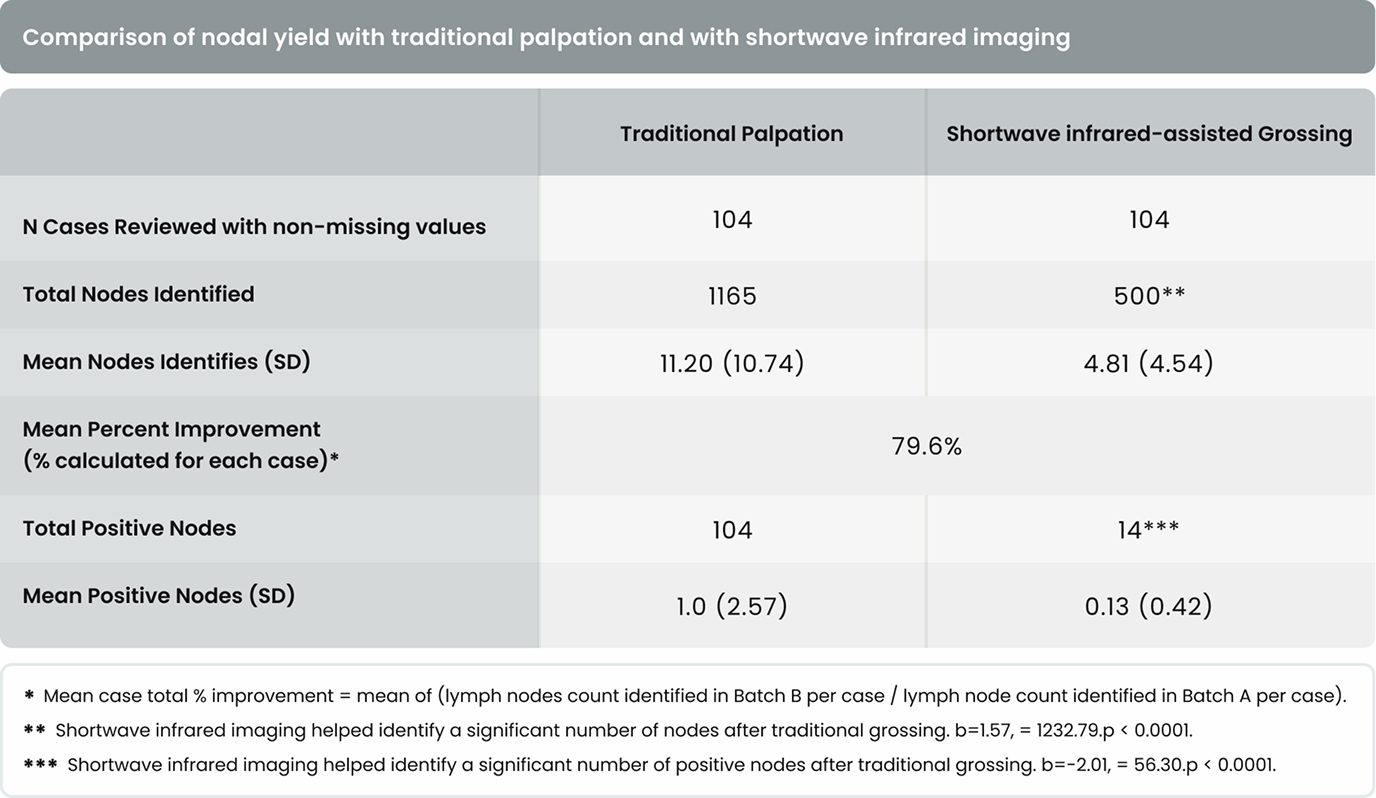
As shown in Table 3, gross examination assisted by shortwave infrared imaging identified a significant number (nonzero) of positive lymph nodes in samples discarded after standard of care lymph node dissection, b = -2.01, X 2= 56.30, p < 0.0001.
As shown in Table 4, in 4 of the 37 cases (10.8%) in Hospital 1 and 7 of the 67 cases (10.4%) in Hospital 2, additional positive lymph nodes were identified with the assistance of shortwave infrared imaging. Overall, in 11 of the 104 cases (10.6%), additional lymph nodes were identified with the assistance of shortwave infrared imaging.
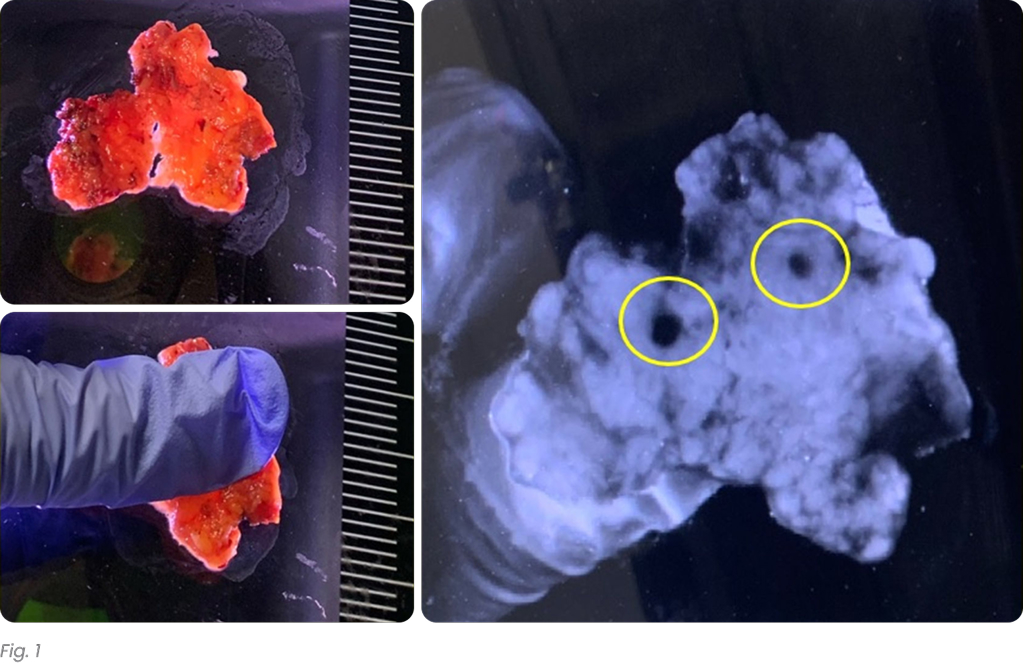
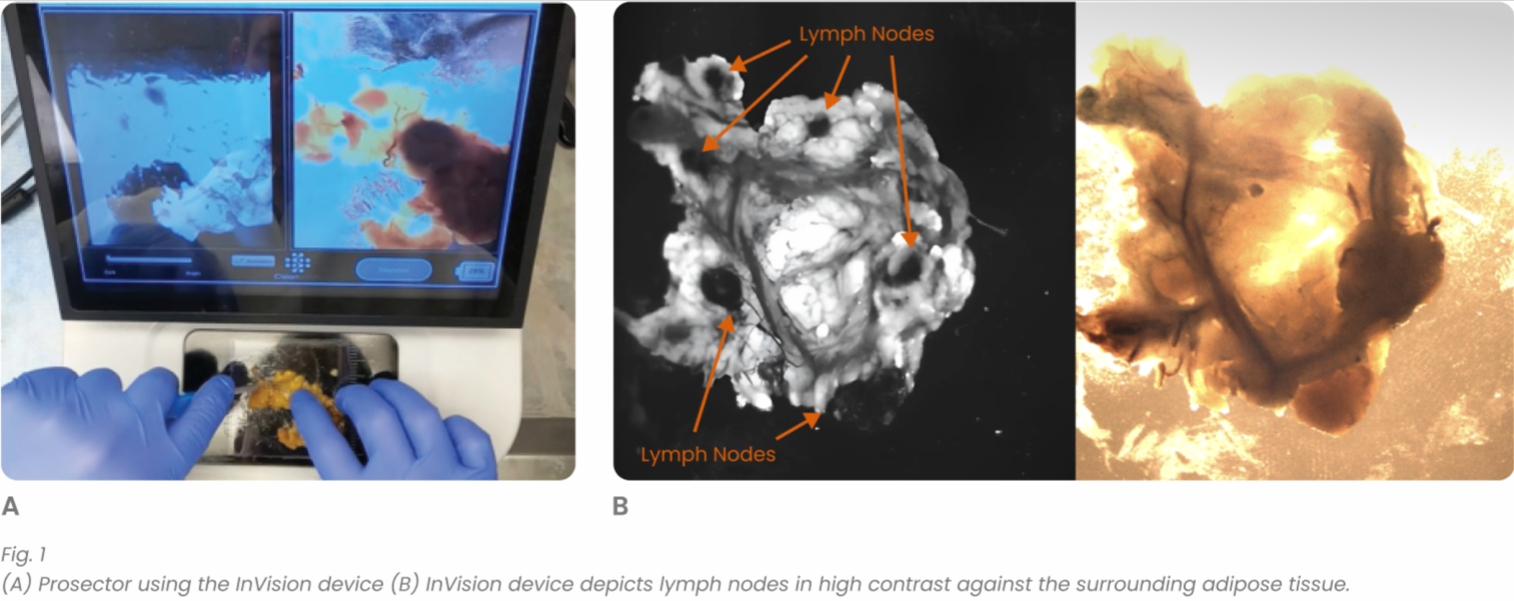
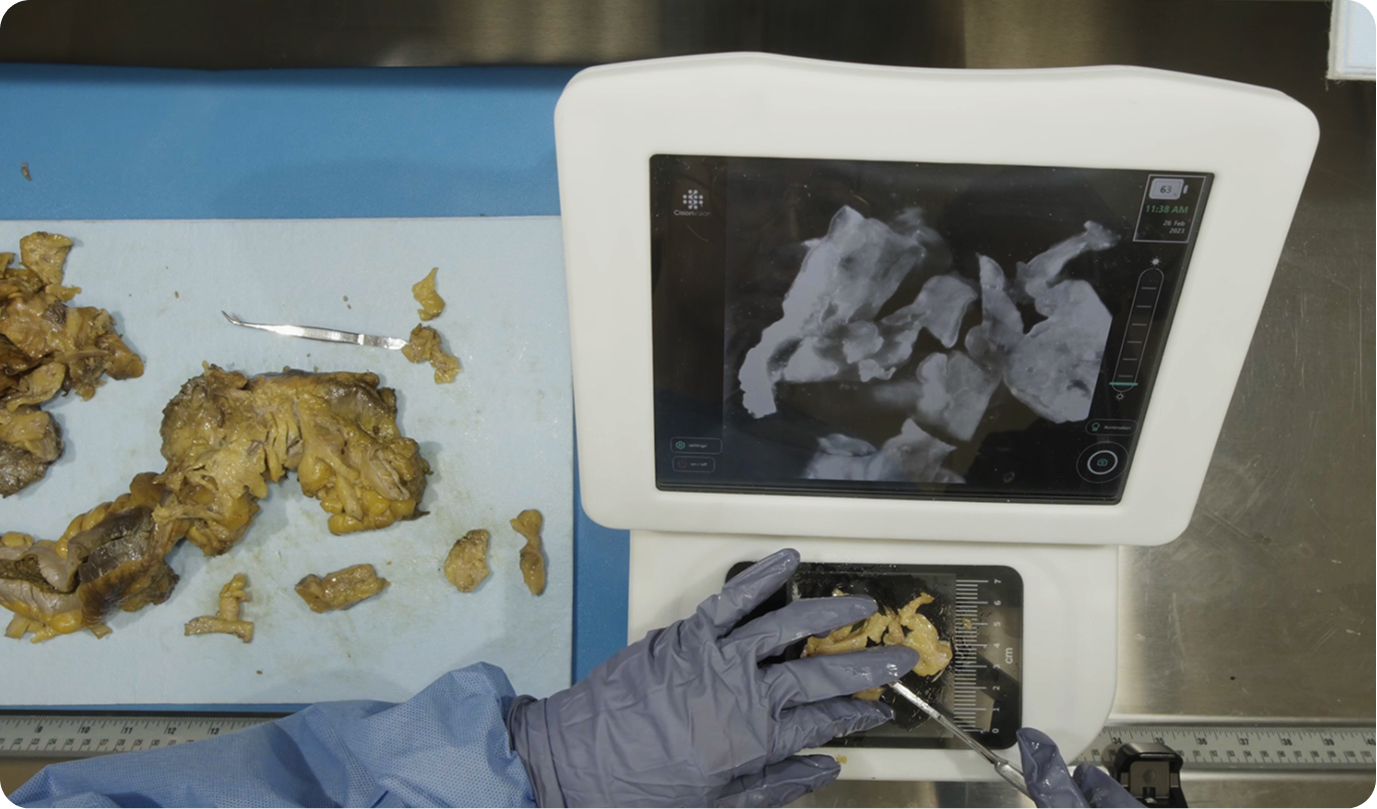
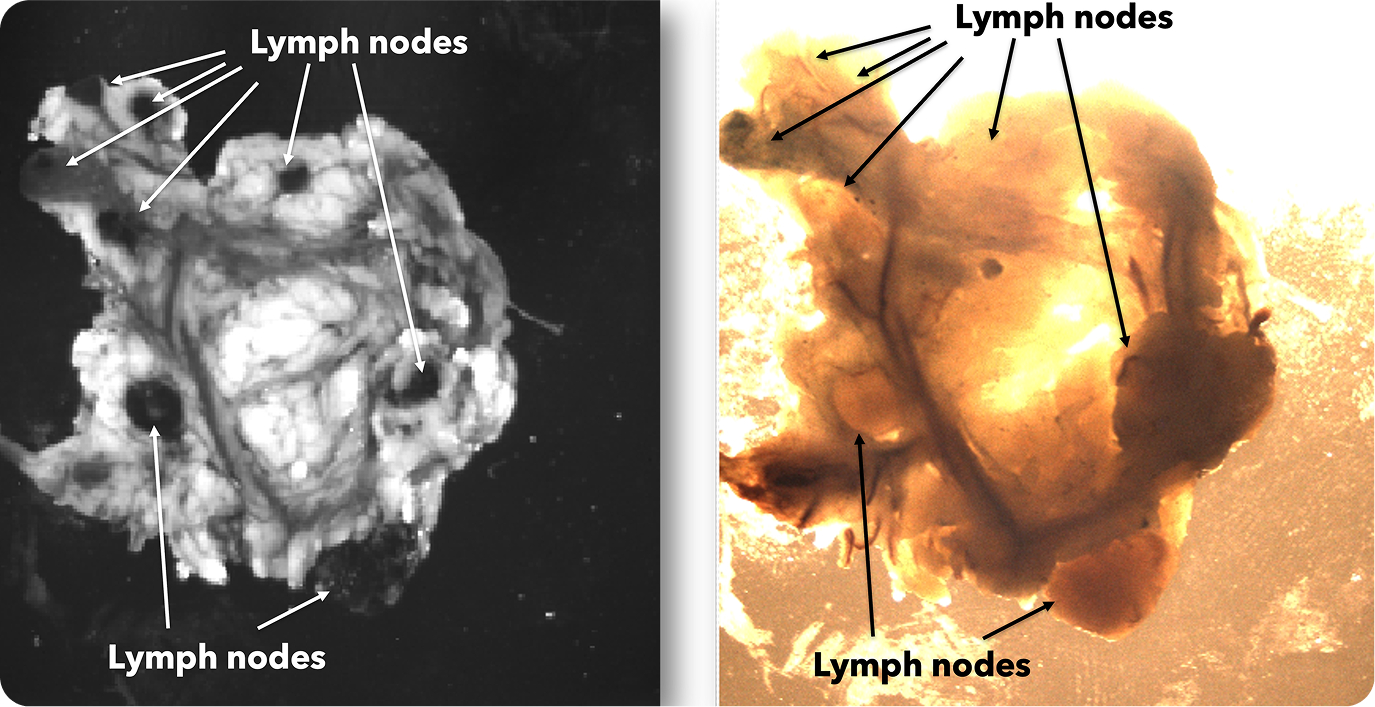
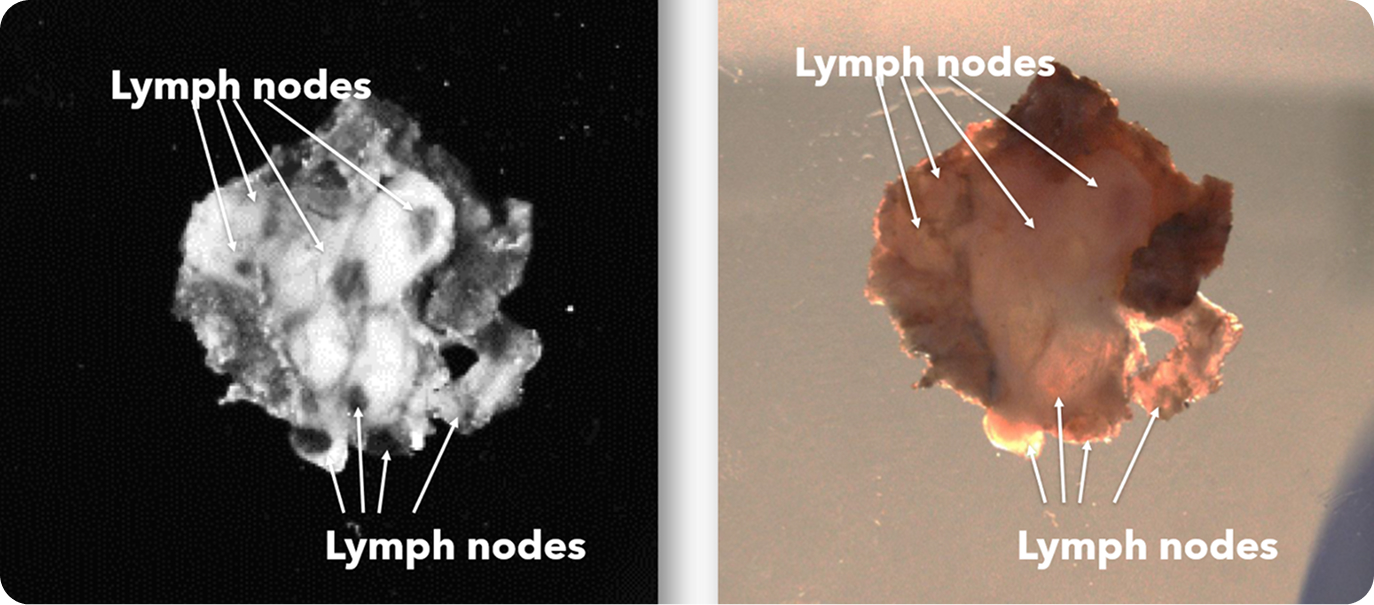
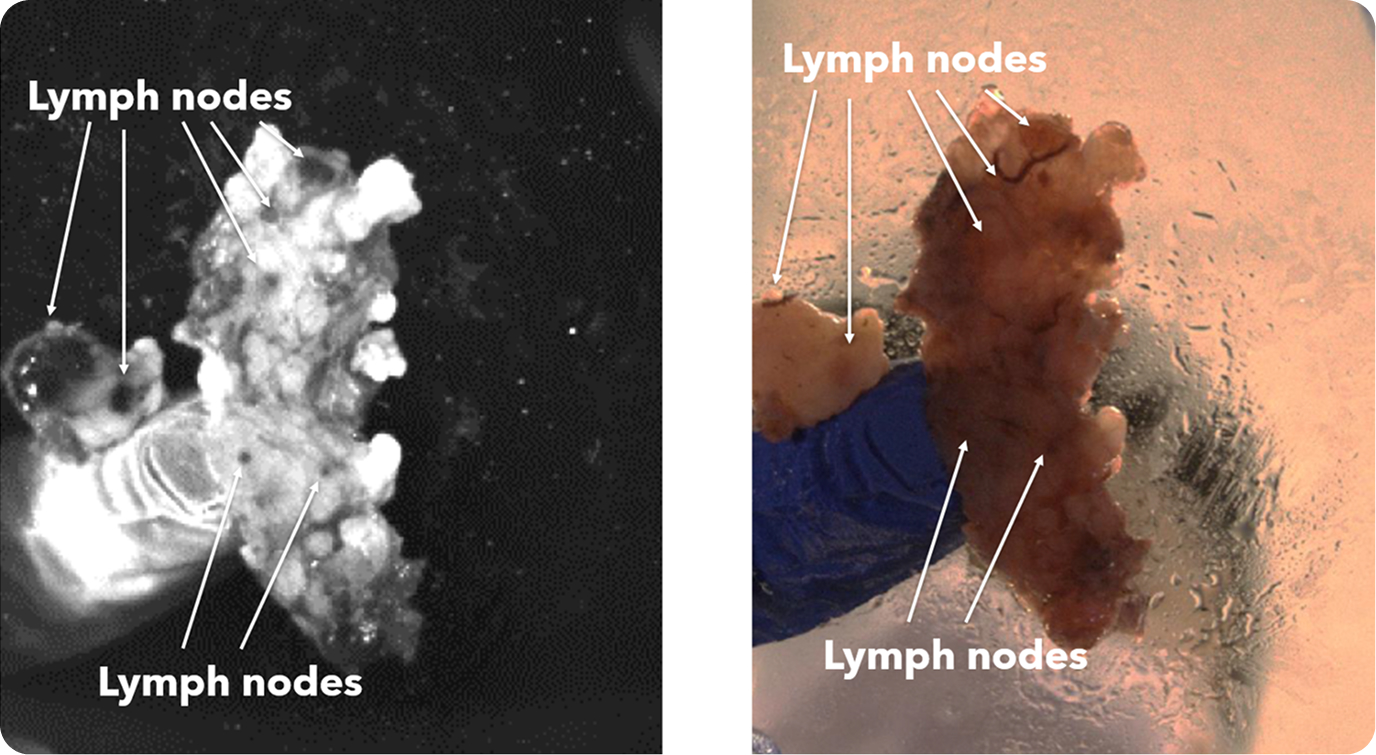
There were 31 lymph node positive cases in total (8 from Hospital 1 and 23 from Hospital 2). In 4 of these positive cases (12.9%), the positive lymph nodes were only discovered by shortwave infrared imaging, with no positive lymph nodes being identified through the standard of care visual and manual dissection. These 4 positive cases would have been wrongly staged as lymph node negative in the absence of shortwave infrared imaging support. An example where the only positive lymph node was identified with the help of shortwave infrared imaging was depicted in Figure 1(B) and (C).
Also, in 11 of the 31 the lymph node positive cases (35.5%), additional lymph nodes were identified with the assistance of shortwave infrared imaging.
The main purpose of this study was to evaluate the effectiveness of shortwave infrared imaging in 1 increasing lymph node counts and 2 identifying additional positive lymph nodes. As shown in the RESULTS section, both hypotheses were strongly supported.
In addition to supporting these two main hypotheses, we also investigated the effectiveness of shortwave infrared imaging for improving compliance of gross examination of colorectal cancer cases. Established guidelines in the United States dictate that colorectal cancer cases yield a minimum of twelve lymph nodes. Failure in reaching twelve lymph nodes after the first round of gross examination results in re-examination of residual adipose tissue, which is time-consuming, labor intensive, and economically costly.15 This problem is particularly common for cases that had been previously treated with radiation or chemotherapy.2 Lymph nodes often become smaller and are thus more challenging to retrieve after such treatments, with reassessment of the gross specimen multiple times being common for such cases. In this study, only 34.1% (29/85) of colorectal cancer cases achieved the required number of 12 lymph nodes after the first round of grossing. Shortwave infrared imaging brought 35.7% (20/56) of the non-compliant cases into compliance, obviating the need to return to the specimen in over a third of these cases.
The data provides compelling evidence for the benefit of shortwave infrared imaging in lymph node dissection in the surgical pathology laboratory. However, as a first large scale multi-center pilot study of this emerging technology, there are several limitations to this study.
The first limitation is the inconsistency in the user proficiency in the technology. When comparing the percentage improvement that resulted from the assistance of shortwave infrared imaging in 1 increasing lymph node counts, 2 increasing positive lymph node counts, and 3 improving compliance, Hospital 1 consistently showed a larger improvement, which could suggest a higher proficiency level of the users in Hospital 1. Because Cision InVision™ Pathology Imaging System does not directly identify lymph nodes (but rather shows a shortwave infrared reflection which is indicative of the natural water content difference between tissue types), the users are responsible for interpreting the images in real time and deciding on which tissue pieces to submit. Robust training and testing protocols for users in a future study can help overcome this limitation.
The second limitation is that the two-step evaluation process is not representative of the practical workflow for using the shortwave infrared imaging system. In a real-world scenario, the first round of gross examination can be carried out together with the assistance of the shortwave infrared imaging system. There is no practical reason for the user to only use the device for searching additional lymph nodes in residual adipose tissue after manual palpation is completed. One can argue that the shortwave infrared imaging system can have an even larger impact in a real-world scenario compared to what has been shown in this study, because the two-step approach places this new technology in a disadvantaged position to demonstrate its effectiveness. Future studies where shortwave infrared imaging is used in the first round of gross examination may be conducted to measure the effectiveness of this new technology in standard practice settings.
The third limitation is that this study was not completely blinded. Although we blinded the pathologists while they review histological results from different batches, there is no way to blind the prosectors with regard to gross examination with or without the device. The psychological factors of the users of the device could have played a role in affecting the numbers of lymph node counts. However, because these procedures were conducted all by experienced board-certified PAs with patients’ best interests in mind, the impact of this role is unlikely to have changed any of the major conclusions that we drew from the data above.
Other potential effects that shortwave infrared imaging can have that were not investigated in this study are 1 time saving in lymph node searches and 2 cost saving for reducing the number of cassettes submitted for histological processing. Future studies need to be conducted to evaluate these potential benefits.
In conclusion, this two-center, 104-patient large-scale pilot study, demonstrated that shortwave infrared imaging helps not only find additional lymph nodes, but also additional positive lymph nodes. Future studies will need to be conducted to overcome the limitations of this study and to investigate other potential benefits of shortwave infrared imaging.